The study in patients with staphylococcal Acute Bacterial Skin and Skin Structure Infections (ABSSSI) demonstrated efficacy and safety of the first in class staphylococcal-selective antibiotic Debio 1450 at the two doses tested
Lausanne, Switzerland – January 10, 2017 – Debiopharm International SA (Debiopharm – www.debiopharm.com), part of Debiopharm Group™, a Swiss-based global biopharmaceutical company, today announced positive results from a large Phase II study of the staphylococcal-selective antibiotic Debio 1450 for the treatment of Acute Bacterial Skin and Skin Structure Infections (ABSSSI).
This multi-center, randomized, double-blind study was designed to compare the efficacy, safety and tolerability of intravenous and oral Debio 1450 at two dose regimens with intravenous vancomycin / oral linezolid as an active comparator in 330 patients with clinically documented ABSSSI due to staphylococci including mostly Staphylococcus aureus sensitive or resistant to methicillin (MSSA or MRSA).
The study objectives were met, demonstrating non-inferiority of Debio 1450 to comparator in all pathogenic staphylococci species infected patient populations including MRSA and ensuring that treatment with Debio 1450 at both doses was safe and well tolerated. The non-inferiority was also consistent across stratification parameters (Cellulitis and Diabetes Mellitus status).
“These topline results are very encouraging and fully support our efforts towards more targeted antibiotics”, said Bertrand Ducrey, CEO of Debiopharm International. “Our data demonstrate that Debio 1450 can be as efficient as broad-spectrum antibiotics currently used. In addition, by selectively targeting the causative pathogen and preserving the microbiota, we expect to improve patients’ quality of life throughout and after the treatment. We will now pursue our development efforts to provide patients and clinicians with this new treatment option for skin and other serious Staphylococcus-related infections”.
About the study
The study was designed as a non-inferiority trial in accordance with the most recent Guidance issued by the Food and Drug Administration (FDA) and was conducted at 25 sites in the United States of America. 330 patients were enrolled in the Trial and randomized across three dosing arms.
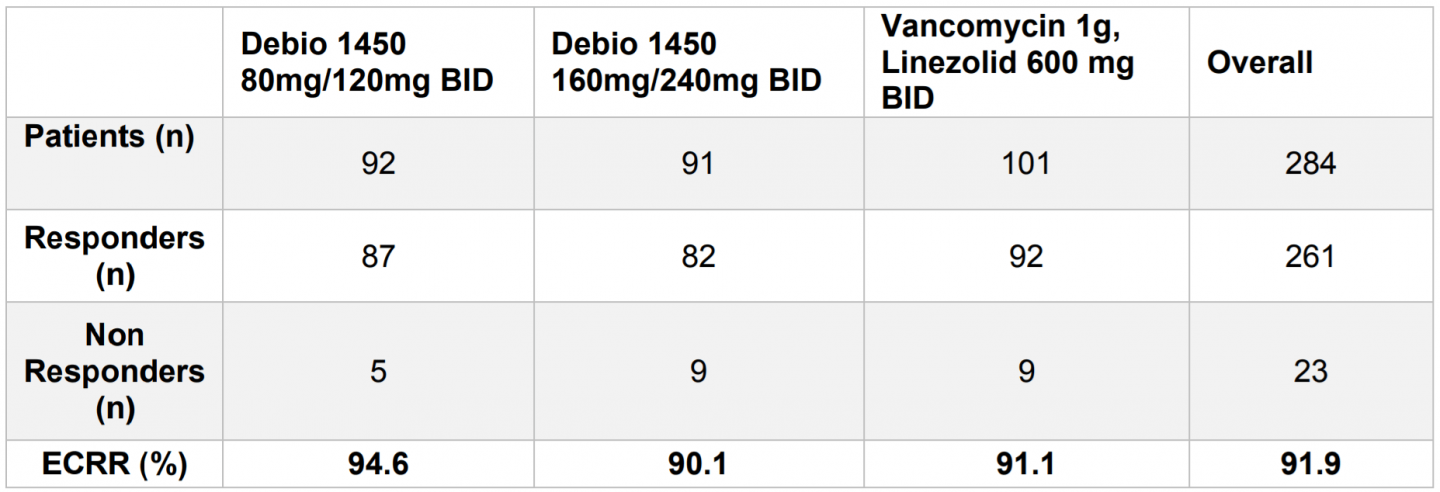
Efficacy analyses were performed on the Microbiological Intent-to-Treat (mITT) population that includes all randomized patients who were culture positive for any staphylococcal species considered pathogenic and received at least one dose of the study drug.
Study Primary Endpoint: Early Clinical Response and Rate (ECRR) at 48 to 72 hours in mITT population
About Debio 1450
Debio 1450 is a new antibiotic benefiting from both oral and IV formulations. It is a highly potent, staphylococcal-selective antibiotic with a low propensity to emergence of resistance. This first-in-class FabI inhibitor retains its activity on staphylococci resistant to antibiotics currently in clinical use including beta lactams, vancomycin, daptomycin or linezolid. Debio 1450 is being developed in ABSSSI and is perfectly suited to tackle several additional hard-to-treat infections caused by staphylococci.
About Debiopharm International SA
Debiopharm Group™ is a Swiss-headquartered global biopharmaceutical group of five companies active in drug development, GMP manufacturing of proprietary drugs, diagnostics tools and investment management. Debiopharm International SA is focused on the development of prescription drugs that target unmet medical needs. The company in-licenses and develops promising drug candidates. The products are commercialized by pharmaceutical out-licensing partners to give access to the largest number of patients worldwide.
For more information, please visit www.debiopharm.com
We are on Twitter. Follow us @DebiopharmNews at http://twitter.com/DebiopharmNews
